76 year old female presenting with a slowly enlarging mass
71-year-old man with an ampullary mass
June 20, 2019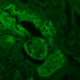
Myeloma cast nephropathy
March 14, 2024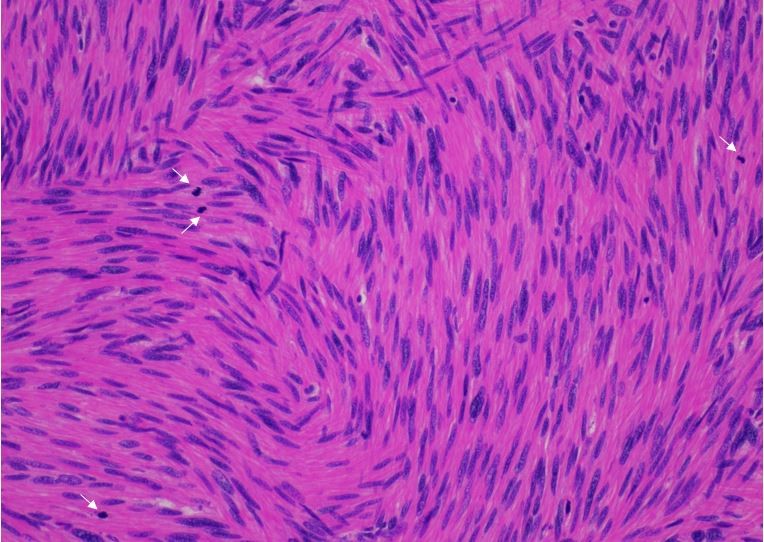
Case submitted by Ridin Balakrishnan, MD under the supervision of Dr. Garrison F. Pease, MD and Dr. Amarpreet Bhalla, MD, FCAP (All from Albert Einstein College of Medicine/Montefiore Medical Center)
Case
A 76 year-old female presented with a slowly enlarging left upper quadrant mass that she had been feeling over the last several months accompanied by mild intermittent left upper quadrant and flank pain. A CT abdomen showed a heterogeneously enhancing, predominantly solid mass within the anteromedial and lower aspect of the left kidney, concerning for neoplasm (Fig. 1). A radical nephrectomy was subsequently performed.
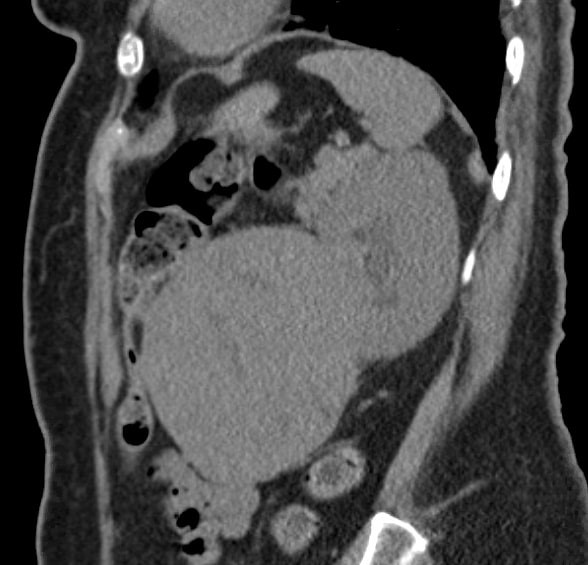
Figure 1: CT Abdomen of the mass in the lower aspect of the Left Kidney
Analysis of the gross specimen revealed a well-circumscribed, un-encapsulated and semi-firm mass present in the lower pole of the kidney that had a white, whorled and fibrous cut surface with multiple focal areas of necrosis (Fig.2). The mass abutted the renal capsule.
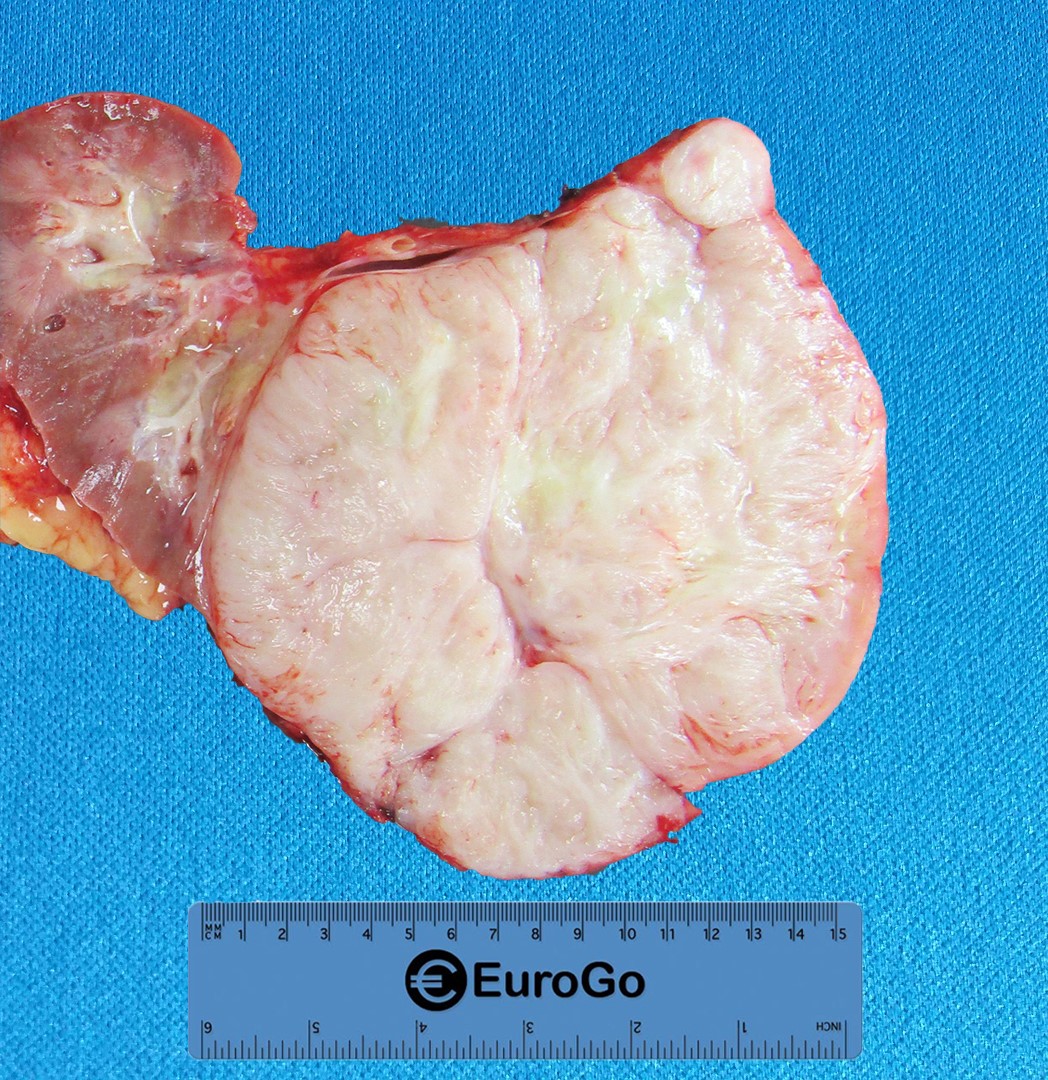
Figure 2: Gross photograph of the mass
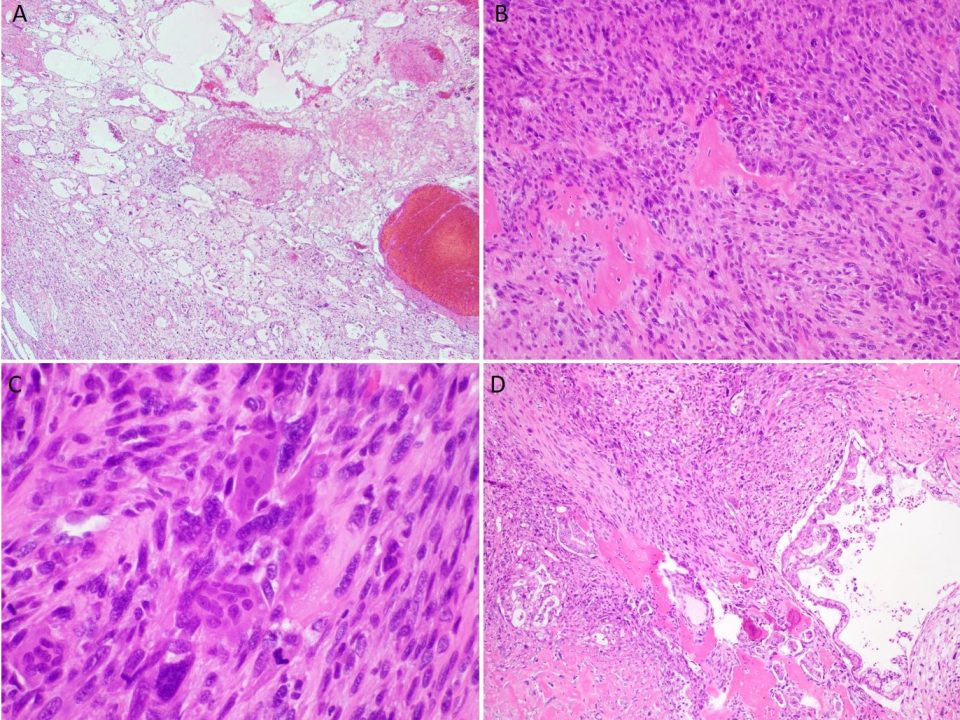
Histologic sections of the mass show intersecting fascicles of malignant spindle cells (Fig. 3a) along with areas of hyalinization and hemorrhage. The tumor cells have eosinophilic cytoplasm, hyperchromatic and blunt-ended cigar-shaped nuclei with a high mitotic index (Fig. 3b). Additionally, some areas are highly pleomorphic with irregular nuclear contours.
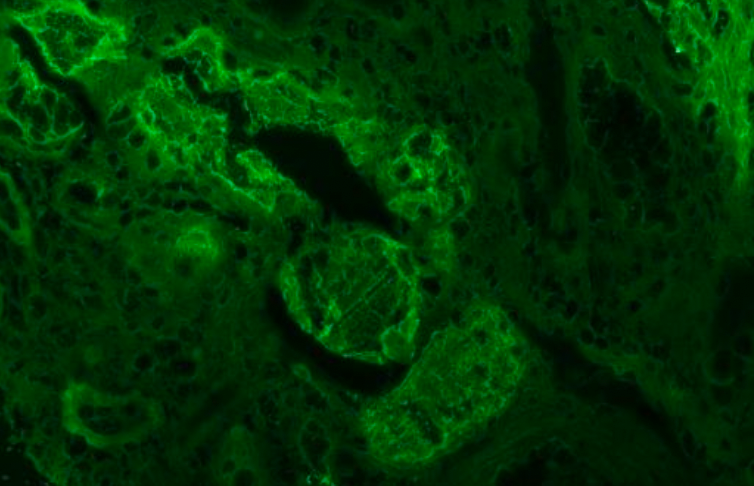
Immunohistochemistry shows positive staining with SMA, Desmin and Caldesmon (Fig. 4) Pancytokeratin, CD99, Bcl-2, CD34, PAX-8, S-100, and HMB-45 were negative.
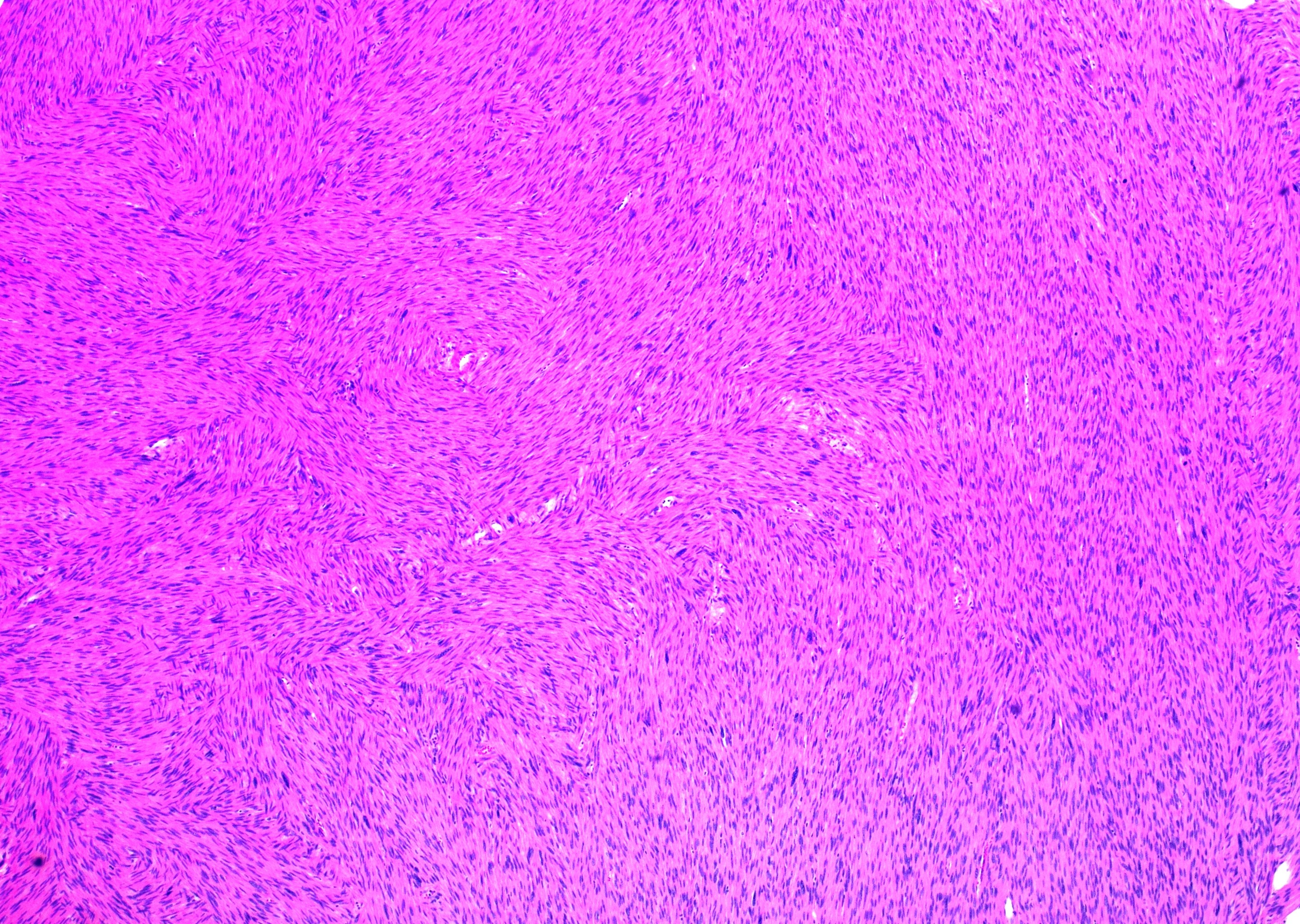
Fig. 3a: H&E stained slide showing intersecting fascicles of spindled cells (40x magnification)

Fig. 3b: H&E stained slide at 200x magnification showing spindled cells with eosinophilic cytoplasm and hyperchromatic and cigar-shaped nuclei. Many mitotic figures are seen (white arrows).

Figure 4: IHC staining of the mass showing diffuse positivity for SMA, Desmin and Caldesmon
Diagnosis and Discussion
The immunomorphologic features of this lesion are consistent with a Renal Leiomyosarcoma, an extremely uncommon entity. While leiomyosarcomas are most common sarcomas in the kidney, sarcomas comprise a very rare frequency of renal tumors; about 0.8-2.7%.1 Renal leiomyosarcomas are usually more common in women and are often clinically indistinguishable from renal cell carcinomas (RCC) as they present with a similar triad of symptoms: abdominal mass, flank pain and hematuria.2 Additionally, primary renal leiomyosarcomas do not have any radiological features that help separate them out from the more common renal malignancies.3
Primary renal leiomyosarcomas may arise from the smooth muscle fibers of the renal parenchyma, renal capsule or the renal vessels.1 Based on where they originate, they may envelop the entire kidney and replace large portions of the parenchyma, often with extrarenal extension. They can also fill the collection system and invade into the renal sinus fat. These tumors are usually solid, white, and well-circumscribed with a whorled cut surface often having areas of cystic degeneration, hemorrhage and necrosis. Microscopy typically shows intersecting fascicles composed of spindled cells that have eosinophilic cytoplasm with hyperchromatic and blunt-ended cigar-shaped nuclei.4 Nuclear pleomorphism is present but variable. Mitotic rates are often very high with atypical forms seen frequently. Areas of coagulative necrosis, myxoid degeneration, perineural and lymphovascular invasion are also commonly observed in these tumors.4 Immunohistochemistry classically shows positive and diffuse cytoplasmic staining with SMA.5 Caldesmon and desmin show variable positivity.6 These tumors are generally negative for pancytokeratin, CD117, ALK1, S100, CD34, HMB-45, MART-1 and p63. Renal leiyomyosarcomas have no known characteristic genetic abnormalities.
While large tumors with high grade features are likely malignant, it is not uncommon to see degenerative atypia, nuclear pleomorphism and occasional mitotic figures in entities such as leiomyomas and angiomyolipomas.8 Other important differential diagnoses for this lesion include sarcomatoid carcinoma with leiomyomatous differentiation and pleomorphic sarcomas.8 Renal leiomyosarcomas are known to have a poor prognosis with a median survival of <2 years and a 5-year survival rate of about 30%.2,7 Complete surgical resection is the only curative therapy and is a predictor of outcome along with grade and tumor size.9 As such, differentiating these from benign and other malignant smooth muscle tumors is crucial and extensive sampling with appropriate IHC characterization staining is warranted to distinguish this rare entity from other similar appearing lesions.
References:
- Makis, William et al. “Primary Renal Leiomyosarcoma Presenting with Subcutaneous and Osseous Metastases: Staging and Follow-Up with 18F-FDG PET/CT.” Nuclear medicine and molecular imaging 52,1 (2018): 69-73. doi:10.1007/s13139-016-0467-0
- Miller JS, Zhou M, Brimo F, Guo CC, Epstein JI. Primary leiomyosarcoma of the kidney: a clinicopathologic study of 27 cases. Am J Surg Pathol. 2010;34:238–242. doi: 10.1097/PAS.0b013e3181cad8c9.
- Valery JR, Tan W, Cortese C. Renal leiomyosarcoma: a diagnostic challenge. Case Rep Oncol Med. 2013;459282
- Samaratunga H., and Delahunt B.: Mesenchymal tumors of adult kidney. Semin. Diagn. Pathol. 2015; 32: pp. 160-171
- Carvalho J.C., Thomas D.G., Lucas D.R. Cluster analysis of immunohistochemical markers in leiomyosarcoma delineates specific anatomic and gender subgroups. 2009;115:4186–4195.
- Watanabe K, Tajino T, Sekiguchi M, Suzuki T. h-Caldesmon as a specific marker for smooth muscle tumors. Comparison with other smooth muscle markers in bone tumors. Am J Clin Pathol. 2000;113(5):663–668.
- Chang A., Brimo F., Montgomery E.A., and Epstein J.I.: Use of PAX8 and GATA3 in diagnosing sarcomatoid renal cell carcinoma and sarcomatoid urothelial carcinoma. Hum Pathol 2013; 44: pp. 1563-1568
- Grignon D.J., Ayala A.G., Ro J.Y., el-Naggar A., and Papadopoulos N.J.: Primary sarcomas of the kidney. A clinicopathologic and DNA flow cytometric study of 17 cases. Cancer 1990; 65: pp. 1611-1618
- Dhawan S, Chopra P, Dhawan S. Primary renal leiomyosarcoma: A diagnostic challenge.Urol Ann. 2012;4(1):48–50. doi:10.4103/0974-7796.91623