Viral Associated Neoplasms of Head and Neck – Presentation Material
September 23, 2018
71-year-old man with an ampullary mass
June 20, 2019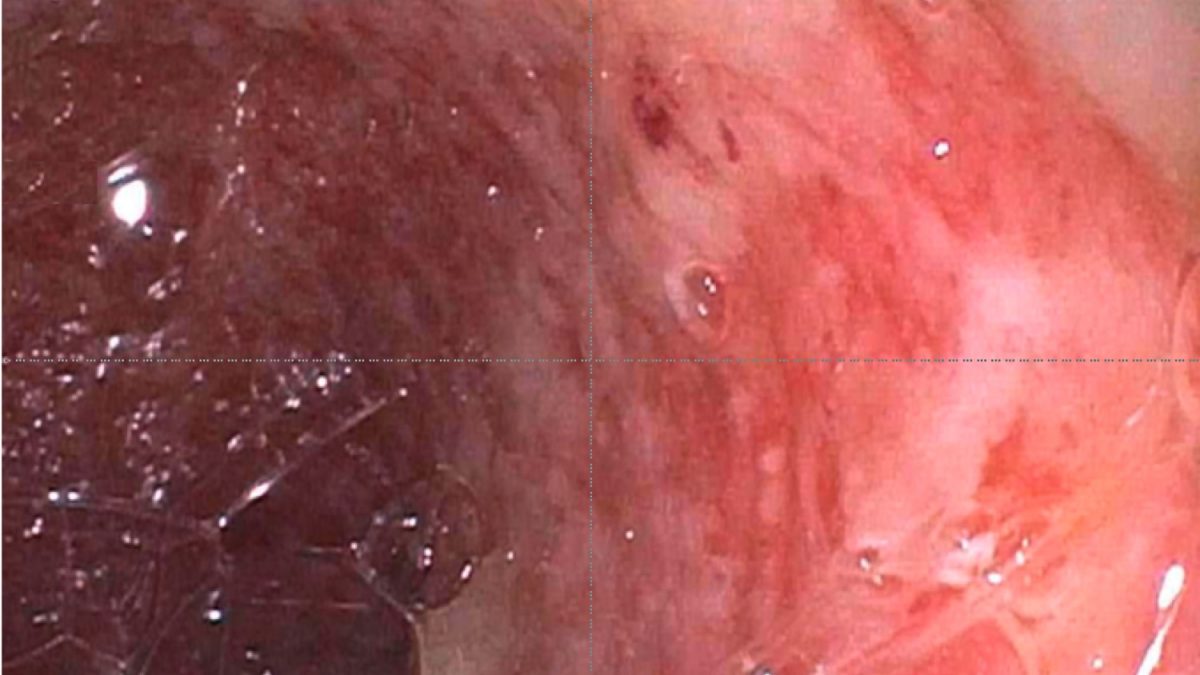
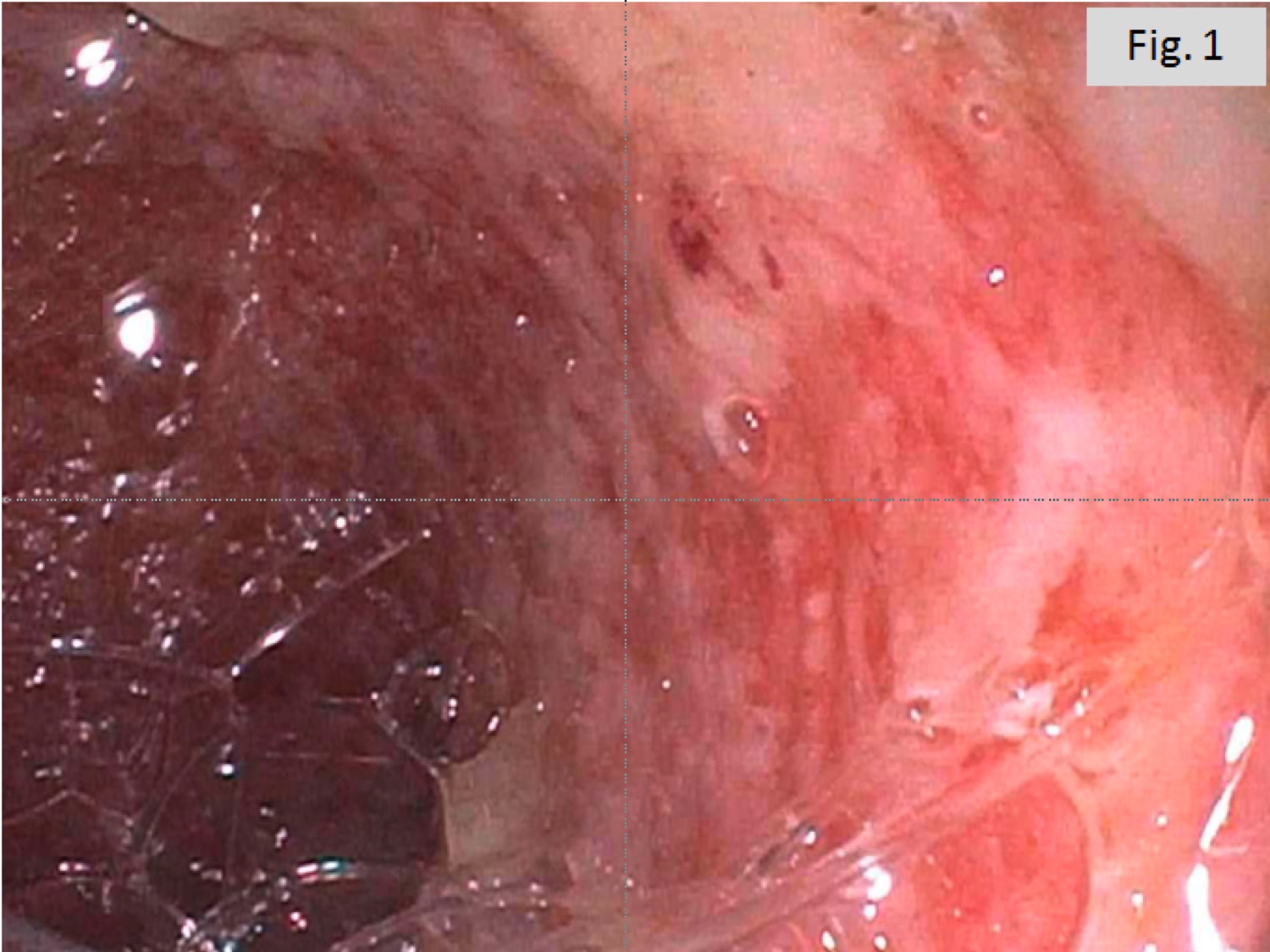
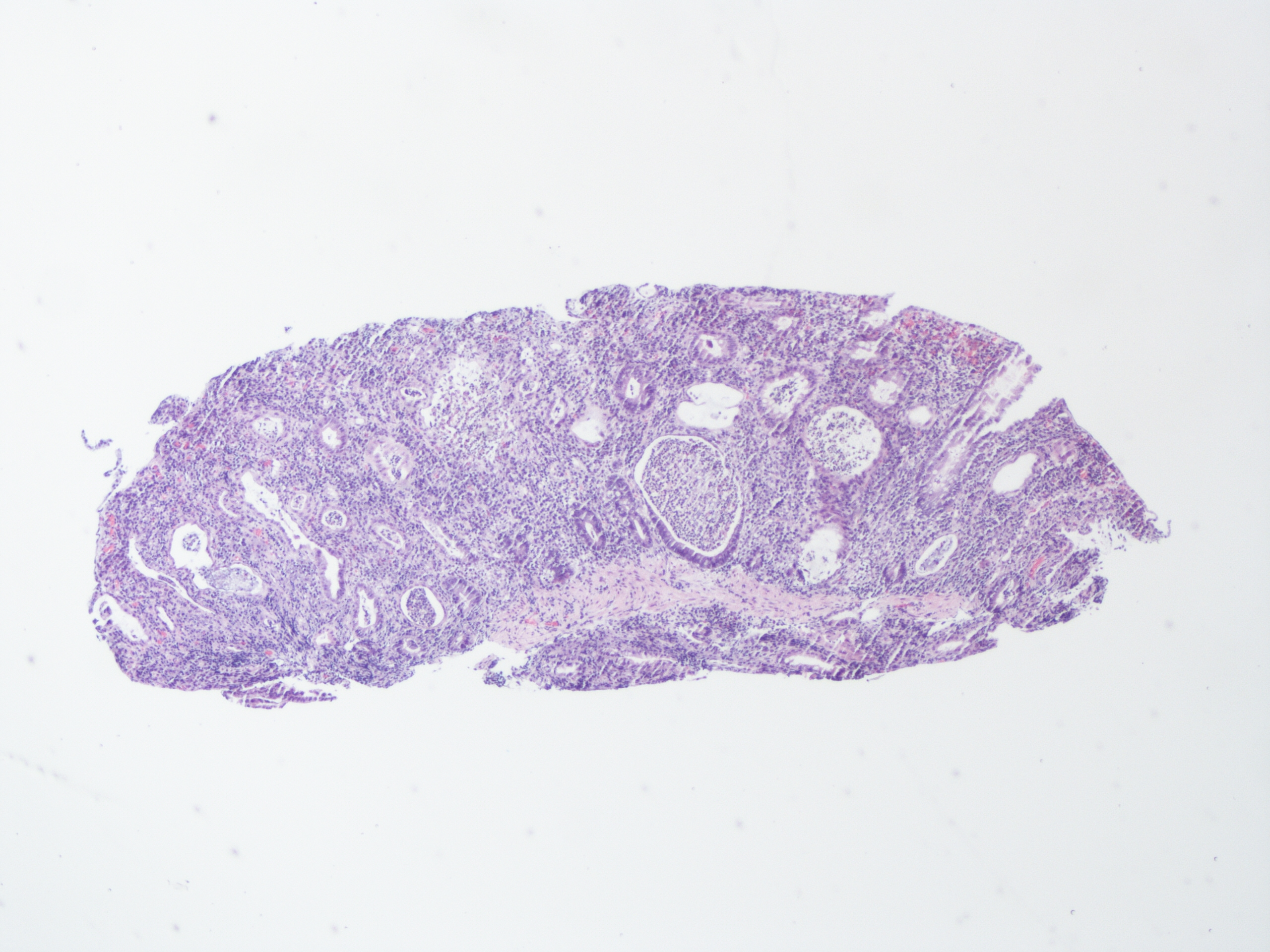
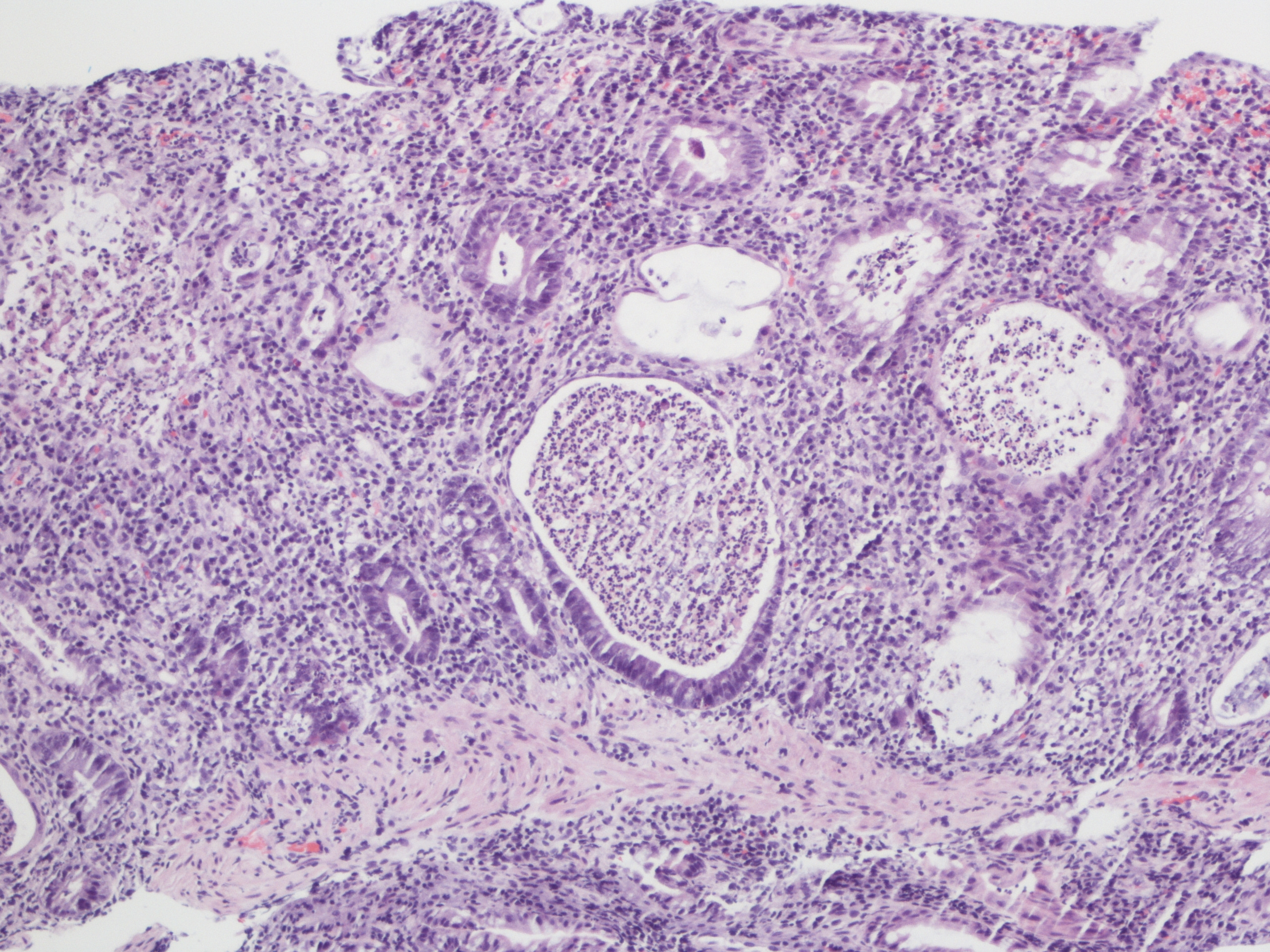
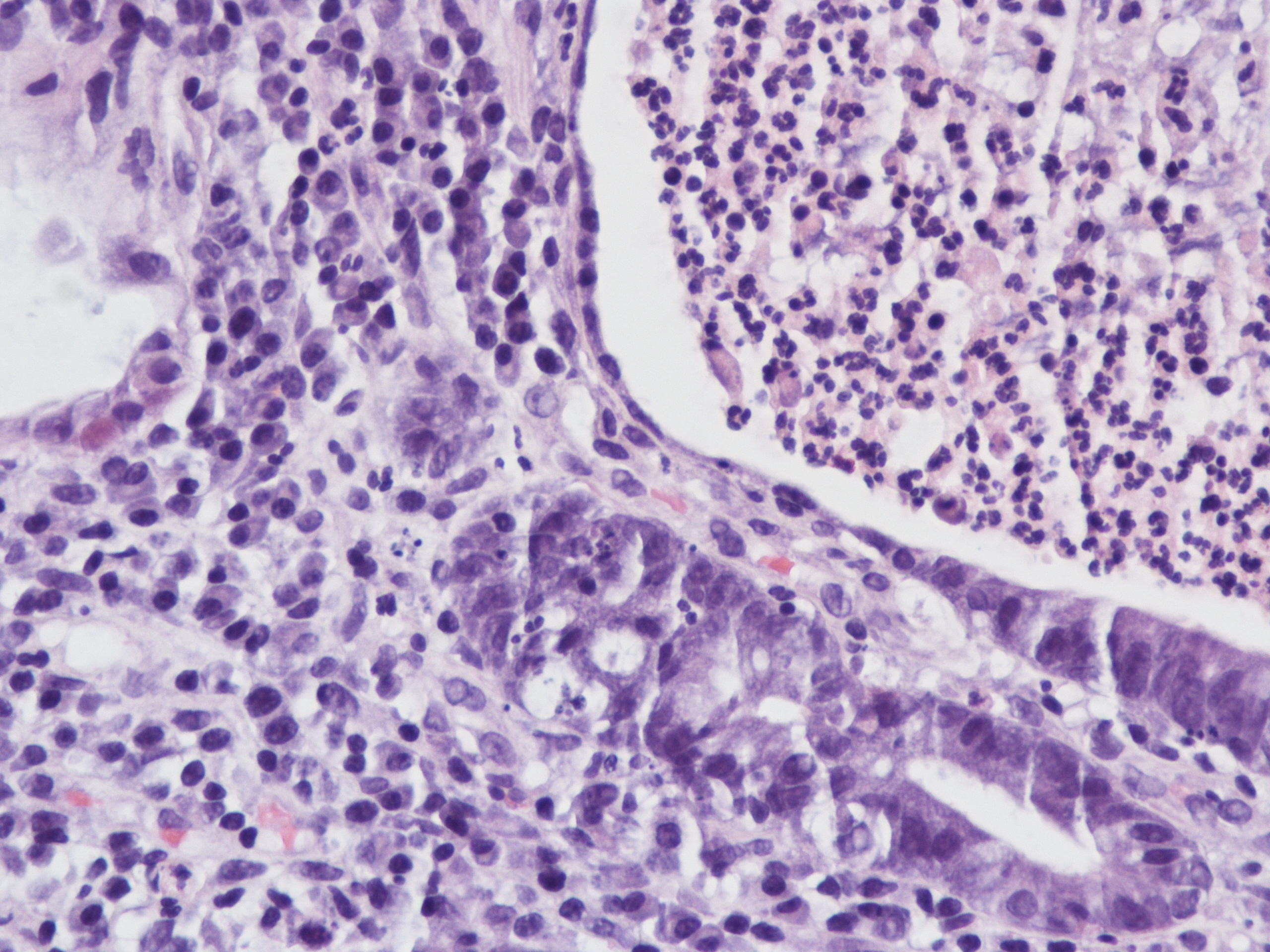
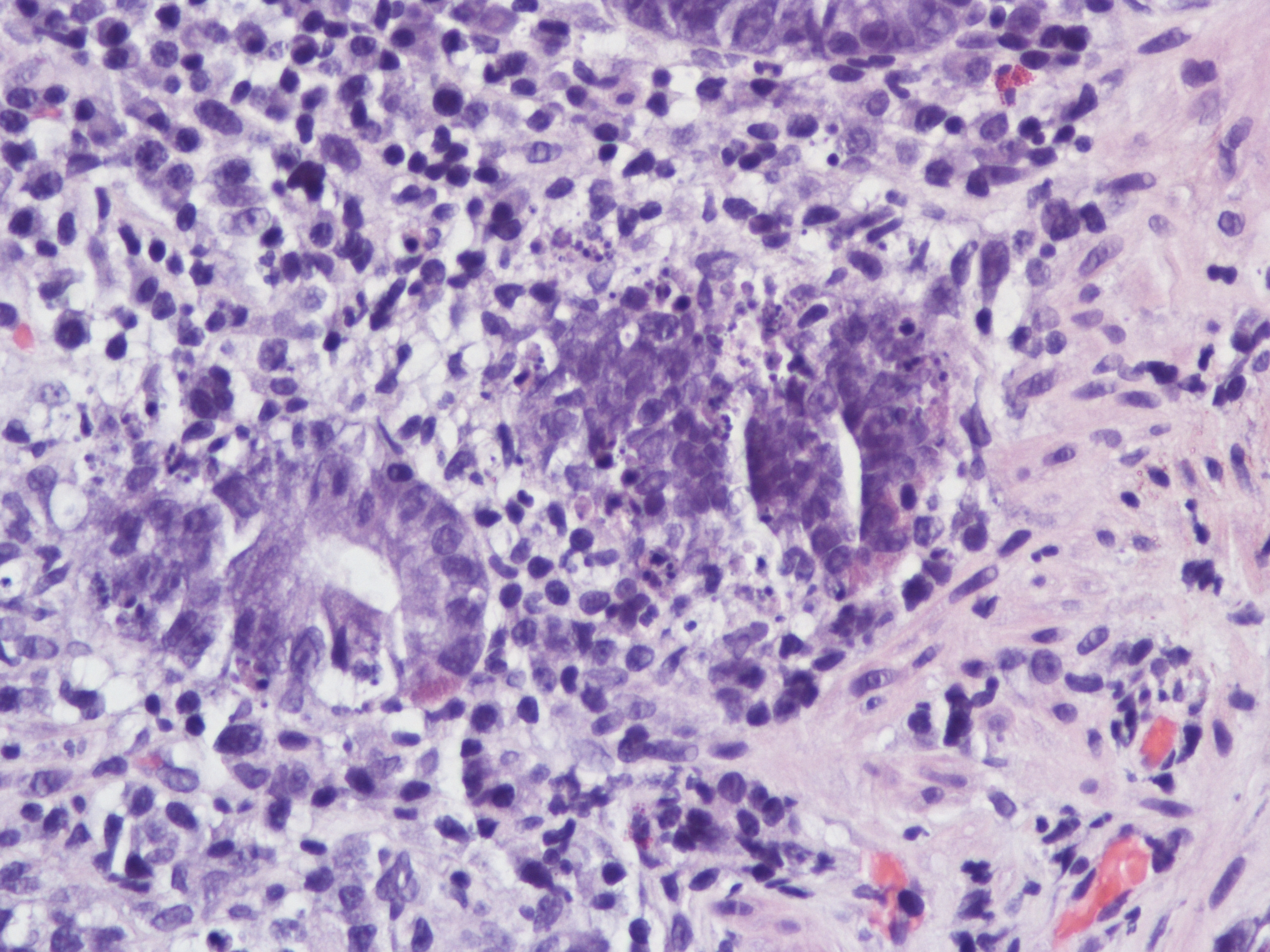
A 60 year old man with history of metastatic lung cancer presented to the emergency room with 6 weeks of copious diarrhea. He had experienced painful cramping in his lower abdomen associated with bowel movements that were bloody, watery, and mixed with mucous. On endoscopy, the distal large bowel mucosa was congested, erythematous, and friable with patches of exudate (Figure 1). A biopsy was performed. On histology, there was an active colitis with neutrophilic infiltration of the lamina propria (Figure 2, H&E 40x magnification), cryptitis, and crypt microabscesses (Figure 3, H&E 100x magnification). A lymphoplasmacytic infiltrate was also identified in the lamina propria. Atrophic appearing crypts with luminal debris and many crypts with prominent crypt apoptosis were identified (Figures 4-5, H&E 400x magnification). No viral cytopathic effects were identified and CMV immunohistochemical stain (not shown) was negative.
In the setting of the patient’s clinical presentation and histologic findings, the differential diagnosis included inflammatory bowel disease (IBD), infection, ischemia, and medication/drug-related injury.
The patient had been treated with Pembrolizumab, an anti-PD-L1 antibody used in the immunotherapy of metastatic lung adenocarcinoma. This drug induces an inflammatory response intended to treat the adenocarcinoma, but has also been reported to cause colitis. Overlapping histologic features of IBD may be seen in such cases. However, the pattern of mucosal injury consisting of atrophic crypts with prominent crypt apoptosis and withering crypts with eosinophils cytoplasm is not a typical feature of IBD. Such pattern should prompt other possible etiologies such as resolving or chronic phase of infection and immunotherapeutic agents.
Interestingly, this patient also had history of Clostridium difficile infection. The pathology report for this case noted that active and resolving phase of Clostridium difficile may have a similar morphological pattern to the high levels of active inflammation seen in this biopsy, raising the possibility of superimposed infection. Therefore correlation with microbiological (particularly stool culture or stool antigen analysis) findings is important.
With the exclusion of other possible etiologies, the most likely diagnosis in this case is anti-PD-L1 associated colitis.
Case submitted by Dr. Yonah Ziemba and Dr. Sujata Sajjan, Zucker School of Medicine at Hofstra / Northwell
References:
Chen JH et al. Histopathologic features of colitis due to immunotherapy with anti-PD-1 antibodies. Am J Surg Pathol 2017;41:643-654. Pubmed Link: https://www.ncbi.nlm.nih.gov/pubmed/28296676
Wang, Yinghong, et al. “Endoscopic and Histologic Features of Immune Checkpoint Inhibitor-Related Colitis.” Inflammatory Bowel Diseases (2018). https://www.ncbi.nlm.nih.gov/pubmed/2971808
Karamchandani, Dipti M., and Runjan Chetty. “Immune checkpoint inhibitor-induced gastrointestinal and hepatic injury: pathologists’ perspective.” Journal of clinical pathology (2018): jclinpath-2018. https://www.ncbi.nlm.nih.gov/pubmed/29703758